Product-IMR Reagent
- Catalog No. MF-TAU-006B
- MOHW-MD-No. 006748
- Taiwan FDA approved for quantitative detection of Tau protein concentration in human plasma to assist in the diagnosis of Alzheimer's disease.
more info
- Catalog No. MF-AB2-006B
- MOHW-MD-No. 006749
- Taiwan FDA approved for quantitative detection of Aβ1-42 concentration in human plasma to assist in the diagnosis of Alzheimer's disease.
more info
- Catalog No. MF-AB0-006B
- MOHW-MD-No. 006750
- Taiwan FDA approved for quantitative detection of Aβ1-40 concentration in human plasma to assist in the diagnosis of Alzheimer's disease.
more info
- Catalog No. MF-TAU-006B
- CE-IVD Certification
- For rapid quantifying Tau protein by Immuno Magnetic Reduction (IMR).
more info
- Catalog No. MF-AB2-006B
- CE-IVD Certification
- For rapid quantifying Amyloid β 1-42 protein by Immuno Magnetic Reduction (IMR).
more info
- Catalog No. MF-AB0-006B
- CE-IVD Certification
- For rapid quantifying Amyloid β 1-40 protein by Immuno Magnetic Reduction (IMR).
more info
- Catalog No. MF-ASC-006B
- CE-IVD Certification
- For rapid quantifying α-Synuclein by Immuno Magnetic Reduction (IMR).
more info
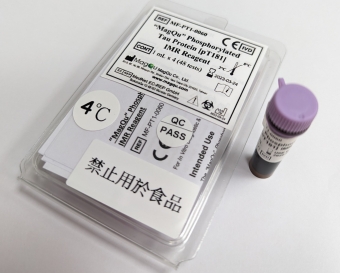
- Catalog No. MF-PT1-0060
- CE-IVD Certification
- For rapid quantifying phosphorylated Tau protein (p-Tau181) by Immuno Magnetic Reduction (IMR).
more info
- Catalog No. MF-NFL-0060
- CE-IVD Certification
- For rapid quantifying Neurofilament light by Immuno Magnetic Reduction (IMR).
more info
Pages